AbstractAlthough a lesion of hidden hearing loss (HHL) has focused on the sprial ganglion, stimulation and recording parameters for auditory brainstem response (ABR) are not yet specific enough to confirm its dysfunction. In the current review, we identify ABR parameters, especially Wave I, for diagnosing HHL through both a systemic review and meta-analysis of 14 articles retrieved from seven journal data bases. The most commonly used parameters were a click or 4 kHz tone burst as the stimulus at a rate of less than 11/sec at more than 80 dB intensity levels with alternating polarity through the ear canal. For quantitative results, our meta-analysis from eight articles demonstrated a significant difference in the overall effect size for amplitude between HHL patients and their counterparts with normal hearing. For the subgroup and its analysis, tone burst and high intensity level revealed significantly lower effect size in HHL patients compared to the control group, whereas the stimuli rate and electrode montage statstically were not statistical significance. Although we examined a small number of studies and their heterogenity, obtaining ABR Wave I was clinically differentiated when noise-induced HHL was considered. We thus suggest that stimulation and recording parameters should be applied to a large number of patients and thereby standardized as sensitive features for Wave I in the clinic.
INTRODUCTIONIt is well known that noise-induced hearing loss (NIHL) is the most common acquired sensorineural hearing loss (World Health Organization, 2015). However, NIHL may not be prominant given continuous exposure of noise even with a moderate level of intensity. Thus, more people might be susceptible to cochlear synaptopathy or so-called hidden hearing loss that is not easilly detected by typical audiological tests (Plack et al., 2014).
Literally, noise-induced ‘hidden’ hearing loss (NIHHL) refers to any functional impairment that is seen in subjects with noise exposing history and no permanent threshold shift (Lin et al., 2011; Shi et al., 2016). This loss is different from the conventional definition of NIHL which is seen in changes of hearing sensitivity or threshold shift (Liberman, 2015; Shi et al., 2016). Although the inner hair cells of the cochlea are relatively insensitive to noise-induced cell death, it has long been recognized that the synapse between inner hair cells and primary spiral ganglion neurons can be damaged by noise (Hu et al., 2020). Further, more recent studies have supported the NIHHL that noise induces damage to both pre- and postsynaptic structures, consequently resulting in permanent loss of the synapses and spiral ganglion neurons (Shi et al., 2016). In the animal studies, it was also revealed that a robust temporary threshold shift elicited by intense noise exposure can result in irreversible/permanent damage to the inner hair cells synaptic connection in the cochlear and auditory nerve fibers (Borg et al., 1995; Lin et al., 2011).
However, since these physiological damages are not accompanied by a permanent shift in hearing threshold, the typical tests might likely miss such a threshold-based assessment. Thus, many contemporary researchers have tried to find accurate and reliable clinical tools to diagnose NIHHL on the suspected patients. Unlike the pure-tone audiometry (PTA), for example, auditory brainstem response (ABR), otoacoustic emission, speech in noise, questionnaire, high frequency PTA were applied by the clinicians as well as researchers. However, despite these efforts, no specific clinical tests have been confirmed as yet that are applicable for diagnosing NIHHL (Bramhall et al., 2017; Fulbright et al., 2017; Grinn et al., 2017).
When looking more closely, of the various tests, Wave I of ABR has been used to inform the function of synapse between inner hair cells and primary spiral ganglion neurons. Some studies found evidence that subjects with noise exposure had reduced amplitdes in the ABR Wave I, a greater summating potential/action potential (SP/AP) ratio in ABR Wave I, a smaller ABR Wave I/V ratio, or increased latency in ABR Wave I (Bramhall et al., 2018; Grose et al., 2017; Lobarinas et al., 2017). However, these reported studies applied to different stimulations and recording parameters of ABR measurement in the methodology, and thus it was difficult to generalize the results and apply them clinically. In this light, we aimed to identify the parameters of ABR, especially Wave I, for diagnosing hidden hearing loss (HHL) while also applying the techniques of systematic review and meta-analysis. Additionally, this study scrutinized specific parameters, such as stimulus type, presentation intensity, polarity, rate, electrode montage, and type of transducer through subgroup analyses. The results of this study will help clinicians more clearly diagnose NIHHL patients in a clinic.
MATERIALS AND METHODOLOGYThe present study was conducted using the Preferred Reporting Items for Systematic Review and Meta-Analysis checklist (Liberati et al., 2009). It also adopted the PROSPERO of Cochrane collaboration (International Prospective Register of Systematic Reviews) methodology, which processed a systematic search for published articles and their reported results.
Search strategyTwo authors (Y. K. & W. H.) independently accessed seven electronic journal databases, namely, CINAHL, Embase, MEDLINE Complete, PubMed Central, Scopus, Science Direct, and Web of Science. While using various key terms combined with the name of diseases, related specific lesions, and their possible clinical tests, the records published from January 2015 to December 2021 were sought and reviewed. The key terms used in this study were: ‘hidden hearing loss’ or ‘cochlear synaptopathy’ or ‘auditory synaptopathy’ and ‘spiral ganglion’ or ‘ribbon synapses’ or ‘normal audiogram’ or ‘normal hearing threshold’ and ‘auditory brainstem response’ or ‘otoacoustic emission’ or ‘auditory steady state response’ or ‘electrocochleography’ or ‘ECochG’ or ‘high frequency audiometry’ or ‘speech in noise’ or ‘speech recognition in noise’ or ‘electrophysiological test’.
Selection of studiesTo determine the main evidence of the related study results, inclusion criteria were applied for the participants, intervention, control, outcome measures, and study design (PICOS). It is better known as the PICOS strategy (Henshaw & Ferguson, 2013). Table 1 displays the five criteria of the PICOS used in this study. We excluded animal studies, no research article (e.g., narrative review paper, conference abstract, letters, book and book chapters, magazines, and proceeding paper), and studies not written in English.
Evaluation of study qualityUnder the modified Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guideline (GRADE Working Group, 2004), the quality of the included studies was evaluated using 10 parameters: randomization, control group, power calculation, blinding, outcome measure reporting, outcome measure, statistical reporting, external validity, reporting of compliance, and consistent evidence. Each parameter was scored from 0 to 2 points, resulting in a total of 20 points as its sum. While reflecting the extent of credibility for the clinical recommendation, the rating system GRADE was classified into four levels: 16~20 points for high quality, 11~15 points for moderate quality, 6~10 points for low quality, and 0~5 points for very low quality.
Data extraction and synthesisWe mainly focused on the ABR responses of NIHHL patients and their interpretations, especially in Wave I. Some data were pre-specified by pilot processing, if any discrepancies existed between the two independent authors (Y. K. & W. H.). Where any instances of non-agreement for the extracted data occurred, the article and the results were jointly revisited until a consensus was reached. Then two authors independently extracted tangible data for the metaanalysis, namely, author, year of publication, details of study design, characteristics of participants (e.g., number, age, and gender), testing conditions for ABR Wave I, such as stimulus, intensity, polarity, rate, electrode montage, and type of transducer, for results interpretation with main significance.
Outcome measurements and statistical analysisThe effect size of the data was calculated using the random effect model with Hedges’ g and a 95% confidence interval (CI). Selection of the random effects was determined based on a conceptual understanding of the presence of population effects within the enrolled studies, rather than using the statistical results of any homogeneity tests.
Heterogeneity among the studies was evaluated using Cochran’s Q-test and Higgins I2-statistics. If an I2 value and p-value were identified as >50% and <0.10, respectively, we classified the heterogeneity of the effect size as being substantial. If the results of the meta-analysis showed a high heterogeneity, a sub-group analysis was then performed using the four key ABR factors (e.g., stimulus, rate, intensity, and electrode montage). Also, any publication bias was identified using funnel plot and the Egger’s regression test. All analyses were preformed at the level of p < 0.05 using the Comprehensive Meta-Analysis Software package (ver. 3.0; Biostat, Englewood, NJ, USA).
RESULTSIncluded studiesInitially, a total of 1,036 records were searched in seven electronic journal databases. After identifying and screening the articles by these authors, 31 articles were retained to confirm their full text at the eligibility stage. When carefully looking at their full text, only 14 out of 31 articles were selected for the meta-analysis because the remaining studies failed to meet the PICOS criteria for certain specific reasons, i.e., no identification of noise exposure, participants with already significant hearing impairment, out of participant criteria, and not conducting the ABR test. Each step is explained in the flow diagram in Figure 1.
Quality assessmentStudy quality was assessed using ten items, i.e., five items for scientific study validity and five items for finding validity criteria. The study was calculated as 0~2 points for each item, resulting in a total of 20 points for the study quality score. Table 2 shows the scores for each item and overall scores for 14 articles. Overall study quality ranged from low to moderate. The lowest score was recorded by Prendergast et al.(2017) and Spankovich et al.(2017) as 9 and 10 points, respectively, whereas both studies by Bhatt and Wang(2019) and Bramhall et al.(2017, 2018) had the highest score at 15 points.
On the one hand, the scientific study quality scored by five items was assessed as 5 points out of a maximum 10 points in the most studies. Participant randomization or where a control group existed was utilized by nine articles. None of the included articles reported blinding. There was also often a lack of information regarding a power calculation to determine appropriate sample size of the participants in the studies of Spankovich et al.(2017) and Stamper and Johnson(2015). As expected, all articles received 2 points for their reporting of outcome measures.
On the other hand, the items for the finding validity criteria had excellent scores. Eleven out of 14 articles were evaluated as 2 points for adequate reporting of all included outcome measures. In the statistical reporting, six out of 14 articles received 2 points and five out of 14 articles obtained 1 point. Half of the articles scored 2 points, and the other articles scored 1 point for their external validity. Ten out of 14 articles scored 2 points for reporting of participant compliance with noise exposure existence, audiometric thresholds, symptoms, and including other drug use status. All 14 articles scored 2 points for evidence consistency.
Study characteristicsIn Table 3, 14 articles are summarized according to the PICOS cireteria, i.e., participants, intervention, controls, outcome measures, study designs. These studies used eight cohort study designs and six diagnostic study designs and were conducted between 2015 and 2019.
The total number of participants was 734 and their ages ranged from 18 to 55 years (mean, 36.5; standard deviation [SD], 26.16). They all met the criteria for noise-induced cochlear synaptopathy, including a normal hearing audiogram and a normal type of tymanometry bilaterally, and reported no history of middle-ear surgery, neurological disorder, head trauma, and ototoxic exposure. The participants also reported having specific noise exposure history by using self-reported and/or questioonnaires (e.g., noise exposure questionnaire, lifetime exposure to noise and solvents questionnaire).
All articles included the results of ABR Wave I amplitude, but only five articles reported the results of Wave I latency. The stimulus type for ABR Wave I used in the studies varied as click and 1, 2, 3, 4, 6 kHz tone burst although they mainly used click and 4 kHz tone burst stimuli. The stimulus rate largely ranged from 7.05 to 91.24 per second, and intensity ranged from 70 to 110 dB. In terms of polarity, 10 studies used alternating, and the other studies used rarefaction polarity. The electrode montages were measured at the mastoid in five articles, the tympanic membrane in one article, the ear lobe in four articles, and the ear canal in six articles. In terms of conclusions, nine studies found no evidence of noise-induced cochlear synaptopathy from Wave I, whilethe results of five studies were consistent with noise-induced cochlear synaptopathy.
The tendancy of ABR Wave I in HHL patientsBased on the previous studies, the latency of ABR Wave I with a click stimulus at a rate of average 11/sec at the ear lobe, tympanic membrane, and mastoid is summarized in Figure 2A. Overal latency was obtained from 1.58 to 2.69 ms (mean, 1.77; SD, 0.35) in general. In the three electrode montage places the latency at the ear lobe was recorded from 1.61 to 1.71 ms (mean, 1.65; SD, 0.06); the mastoid ranged from 1.6 to 2.69 ms (mean, 1.95; SD, 0.51), and the tympanic membrane ranged from 1.58 to 1.64 ms (mean, 1.61; SD, 0.04).
When using click and a 4 kHz tone burst stimuli at an average 11/sec, amplitude distribution of the ABR Wave I is shown in Figure 2B. Overal Wave I amplitude ranged from 0.05 to 0.769 μV (mean, 0.39; SD, 0.22), while including the amplitude ranged from 0.09 to 0.769 μV (mean, 0.4; SD, 0.21) for the click stimulus and ranged from 0.05 to 0.738 μV (mean, 0.38; SD, 0.26) for the 4 kHz tone burst.
Meta-analysis outcomesFor the meta-analysis, only eight cohort studies were included. Unfortunately, six diagnostic studies were excluded because of having either no pre-post results or no control group. A total of 378 subjects were classified into two groups: 183 subjects for the experimental group and 195 subjects for the control group. Their ages ranged from 18 to 40 years.
Statistically, the ABR Wave I amplitude was significantly lower in the experimental group than in the control group (g = -0.829; 95% CI, -1.594 to -0.065; p = 0.033) (Figure 3A). However, there was still a substantial amount of heterogeneity between the studies (I2 = 91.479%, p = 0.000).
Publication biasTo verify publication bias for the studies included, visual confirmation of symmetry through the funnel plot (Figure 3B), and an Egger’s regression analysis was performed for a statistical analysis of asymmetry. The results indicated no publication bias was found (t = 0.17425, df = 6, p = 0.8674).
Subgroup analysis: stimulus type, rate, intensity, and electrode montageAll the studies were subjected to subgroup meta-analysis according to the ABR parameters. The results of the forest plot are as shown in Figure 4 and the statistical summary is presented in Table 4.
In terms of stimulus type, the effect size was significantly lower in the experimental group than in the control group (g = -0.846; 95% CI, -1.648 to -0.044; p = 0.039). In more detail, the click stimulus showed no significant difference between the experimental group and the control group (g = -0.592; 95% CI, -1.486 to 0.284; p = 0.185). However, when applying the tone-burst stimulus, the experimental group had a significantly lower effect size than the control group (g = -1.505; 95% CI, -2.985 to -0.025; p = 0.046).
The studies were classified into three groups for the stimulus rate: one study for high rate, four studies for moderate rate, and three studies for low rate. The effect size of the subgroup by overall rate indiated no significant difference between the experimental group and the control group (g = -1.147; 95% CI, -2.604 to 0.309; p = 0.123). For instance, the moderate rate (g = -0.782; 95% CI = -1.773 to 0.209; p = 0.122) and the low rate (g = -0.193; 95% CI, -1.336 to 0.949; p = 0.740) showed no significant difference in effect size between the experimental and the control groups. Remakably, the stimulus with the high rate was significantly lower in the experimental group compared to the control group (g = -3.266; 95% CI, -5.415 to -1.118; p = 0.003).
For the presentation level, five studies and three studies had high and low intensity, respectively. The effect size of overall intensity was significantly lower in the experimental group than in the control group (g = -0.832; 95% CI, -1.652 to -0.013; p = 0.047). In more detail, high intensity had a significantly lower effect size in the experimental group when compared to the control group (g = -1.056; 95% CI, -2.095 to -0.018; p = 0.046), but the subgroup with low intensity showed no significant difference between the two groups (g = -0.462; 95% CI, -1.797 to 0.872; p = 0.497).
In the electrode montage, four studies of the ear canal, two studies of the ear lobe, and two studies of the mastoid groups were included. The effect size of the overall electrode montage indicated no significant difference between the experimental group and the control group (g = -0.647; 95% CI, -1.944 to 0.649; p = 0.328). When analyzed in more detail, the ear canal was significantly lower in the experimental group than the control group (g = -1.585; 95% CI, -2.615 to -0.556; p = 0.003). But, the subgroups by ear lobe (g = -0.519; 95% CI, -1.966 to 0.927; p = 0.482) and mastoid (g = 0.394; 95% CI, -1.072 to 1.860; p = 0.599) showed no significant difference between those two groups.
DISCUSSIONSThe main features of NIHHL are the degeneration of nerves caused by damage to synapses, damage to auditory nerve fibers in the internal mother cells, and loss of Schwann cells (Liberman et al., 2015). As mentioned earlier, NIHHL may not affect hearing sensitivity, especially pure-tone thresholds although the patients did complain of difficulty when distinguishing and understanding speech sounds in noise situations. These issues include symptoms caused by problems with more central auditory organs rather than the peripheral parts of auditory pathway and can, therefore, be affected by genetic and psychological factors (tinnitus or hyperacusis) (Kujawa & Liberman, 2015; Stephens et al., 2003). Regardless, the exact method for a precise diagnosis of NIHHL has yet to be found.
As a clinical guide, the aim of the present study was to investigate stimulation and recording parameters of ABR, especially Wave I for diagnosing NIHHL using a systematic review and meta-analysis. Systematic review of the present study indicated that the most commonly used parameters were click or 4 kHz tone burst stimulus at a rate of less than an average 11/sec at more than 80 dB intensity levels with alternating polarity at the ear canal, mastoid, and ear canal. Looking back, Figure 2 infers through examination of the raw data of previous experiments that when conducting the ABR test with click stimulus at a rate of average 11/sec, clinicians are expected to obtain Wave I latency from 1.58 to 2.69 ms although there is a slight difference of latency between the place of electrode montage. Considering that the average absolute latency of Wave I in normal adults is 1.54 ± 0.08 ms, it can be judged that the Wave I latency of NIHHL patients is slightly delayed. In addition, when using click and 4 kHz tone burst stimuli at an average 11/sec, amplitude distribution of ABR Wave I might range from 0.05 to 0.769 μV. However, only six articles reported any findings consistent with noise-induced cochlear synaptopathy or NIHHL.
From the results of current meta-analysis, Wave I amplitude of ABR was significantly lower in NIHHL patients compared to the control group, although there was substantial heterogeneity. Bramhall et al.(2017) and Valderrama et al.(2018) reported evidence of reduced ABR Wave I amplitude in high-noise exposure groups. Similarly, Stamper and Johnson(2015) found that only female participants with more noise exposure showed significantly reduced amplitude in the ABR Wave I. Liberman et al.(2016) found that particiapnts with high noise exposure background had an elevated ABR Wave I SP/AP ratio. Also, Grose et al.(2017) confirmed reduced ABR Wave I amplitude and smaller ABR Wave I/V ratio in the noise exposure group.
Further still, subgroup meta-analysis supported that stimulation and recording parameters via a tone-burst stimulus, high rate, high intensity, and ear canal may be useful for applying the NIHHL patents. Nevertheless, there are certain limitations that should be addressed in future studies. First even though our results might inform the clinicians, small numbers of studies can limit the integrity of the analysis. Moreover, only six articles reviewed reported significant results for the noise-induced cochlear synaptopathy. To be precise, any future study should be analyzed using a large number of studies based on the stimulation and recording parameters suggested in the present study. Secondly, we need to find the most sensitive factors among all the parameters so as to clinically differentiate patients between suspected patients or those that are not suspected using developed NIHHL sensitive features.
Taken all together, ABR Wave I can be one of the key diagnostic tools used to diagnose function/dysfunction of the spiral ganglion neurons for patients with cochlear synaptopathy or hidden hearing loss because this test is by far the most valid and the most common test for clinicians to use in the clinic.
NotesFunding This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2022S1A5A2A01044793). Author Contributions Conceptualization: Woojae Han. Data curation and Formal analysis: Yeoju Kim, Woojae Han. Funding acquisition: Woojae Han. Methodology: Yeoju Kim. Project administration: Woojae Han. Visualization: Yeoju Kim. Writing—original draft: Yeoju Kim. Writing —review & editing: Woojae Han. Figure 1.Preferred Reported Items for Systematic Reviews and a Meta-Analysis flow diagram using inclusion and exclusion criteria. PICOS: participants, intervention, control, outcome measures, and study, ABR: auditory brainstem response. 
Figure 2.Latency of the auditory brainstem response (ABR) Wave I with a click stimulus at a rate of average 11/sec at the ear lobe, tympanic membrane, and mastoid (A) and amplitude distribution of ABR Wave I between a 4 kHz tone-burst, and a click stimulus at an average of 11/sec (B). 
Figure 3.Forest plot comparing the auditory brainstem response Wave I amplitude for the experimental group and control group (A) and the funnel plot for standard error by Hedges’g (B). 
Figure 4.A forest plot for subgroup meta-analysis based on stimulus type (A), by rate (B), by intensity (C), by electrode montage (D). 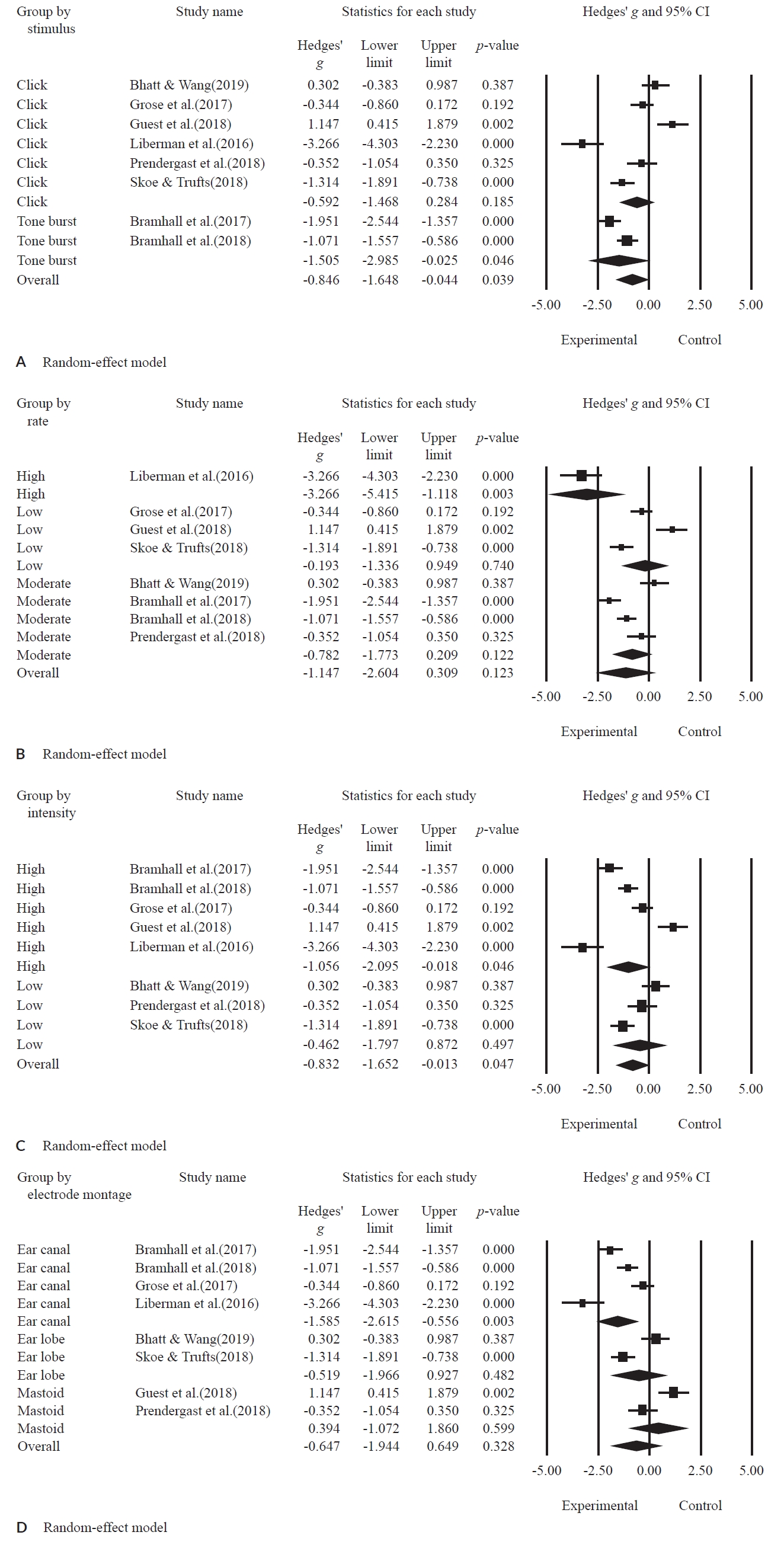
Table 1.Inclusion criteria for the present study based on participants, intervention, control, outcomes, and the study design (PICOS) strategy Table 2.Study validity criteria, study quality scores, and levels of evidence of the fourteen articles included in the present study Criteria score: 0 = flawed or no information from which to make a judgement; 1 = weak information, incorrect use or lack of detail from which to make a judgment; 2 = appropriate use and reporting. Study quality score: the sum of scores for scientific study and finding validity criteria, Level of evidence: study quality score of 0~5 = very low; 6~10 = low; 11~15 = moderate; 16~20 = high (adapted from GRADE Working Group, 2004) Table 3.Demographic information and clinical characteristics of the enrolled 14 studies and recording parameters and the main finding of auditory brainstem responses for Wave I in noise-induced hidden hearing loss Table 4.Statistical summary of the subgroup meta-analysis results according to stimulation and recording parameters: stimulus type (click and tone burst); rate (high, moderate, low); intensity (high, low); electrode montage (ear canal, ear lobe, mastoid)
REFERENCESBhatt, I. S. & Wang, J. (2019). Evaluation of dichotic listening performance in normal-hearing, noise-exposed young females. Hearing Research, 380, 10-21.
Borg, E., Canlon, B., & Engstrom, B. (1995). Noise-induced hearing loss. Literature review and experiments in rabbits. Morphological and electrophysiological features, exposure parameters and temporal factors, variability and interactions. Scandinavian Audiology, Supplementum, 40, 1-147.
Bramhall, N. F., Konrad-Martin, D., & McMillan, G. P. (2018). Tinnitus and auditory perception after a history of noise exposure: Relationship to auditory brainstem response measures. Ear and Hearing, 39(5), 881-894.
Bramhall, N. F., Konrad-Martin, D., McMillan, G. P., & Griest, S. E. (2017). Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear and Hearing, 38(1), e1-e12.
Fulbright, A. N. C., Le Prell, C. G., Griffiths, S. K., & Lobarinas, E. (2017). Effects of recreational noise on threshold and suprathreshold measures of auditory function. Seminars in Hearing, 38(4), 298-318.
GRADE Working Group. (2004). Grading quality of evidence and strength of recommendations. British Medical Journal, 328(7454), 1490.
Grinn, S. K., Wiseman, K. B., Baker, J. A., & Le Prell, C. G. (2017). Hidden hearing loss? No effect of common recreational noise exposure on cochlear nerve response amplitude in humans. Frontiers in Neuroscience, 11, 465.
Grose, J. H., Buss, E., & Hall, J. W. (2017). Loud music exposure and cochlear synaptopathy in young adults: Isolated auditory brainstem response effects but no perceptual consequences. Trends in Hearing, 21, 2331216517737417.
Guest, H., Munro, K. J., Prendergast, G., Millman, R. E., & Plack, C. J. (2018). Impaired speech perception in noise with a normal audiogram: No evidence for cochlear synaptopathy and no relation to lifetime noise exposure. Hearing Research, 364, 142-151.
Henshaw, H. & Ferguson, M. A. (2013). Efficacy of individual computer-based auditory training for people with hearing loss: A systematic review of the evidence. PLoS One, 8(5), e62836.
Hu, N., Rutherford, M. A., & Green, S. H. (2020). Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca2+-permeable AMPA receptors. Proceedings of the National Academy of Sciences of the United States of America, 117(7), 3828-3838.
Kujawa, S. G. & Liberman, M. C. (2015). Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research, 330(Pt B), 191-199.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100.
Liberman, L. D., Suzuki, J., & Liberman, M. C. (2015). Dynamics of cochlear synaptopathy after acoustic overexposure. Journal of the Association for Research in Otolaryngology, 16(2), 205-219.
Liberman, M. C., Epstein, M. J., Cleveland, S. S., Wang, H., & Maison, S. F. (2016). Toward a differential diagnosis of hidden hearing loss in humans. PLoS One, 11(9), e0162726.
Lin, H. W., Furman, A. C., Kujawa, S. G., & Liberman, M. C. (2011). Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology, 12(5), 605-616.
Lobarinas, E., Spankovich, C., & Le Prell, C. G. (2017). Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR Wave - I amplitude reductions. Hearing Research, 349, 155-163.
Plack, C. J., Barker, D., & Prendergast, G. (2014). Perceptual consequences of “hidden” hearing loss. Trends in Hearing, 18, 2331216514550621.
Prendergast, G., Guest, H., Munro, K. J., Kluk, K., Léger, A., Hall, D. A., et al. (2017). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hearing Research, 344, 68-81.
Prendergast, G., Tu, W., Guest, H., Millman, R. E., Kluk, K., Couth, S., et al. (2018). Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hearing Research, 364, 38-47.
Shi, L., Chang, Y., Li, X., Aiken, S., Liu, L., & Wang, J. (2016). Cochlear synaptopathy and noise-induced hidden hearing loss. Neural Plasticity, 2016, 6143164.
Skoe, E. & Tufts, J. (2018). Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans. Hearing Research, 361, 80-91.
Spankovich, C., Le Prell, C. G., Lobarinas, E., & Hood, L. J. (2017). Noise history and auditory function in young adults with and without type 1 diabetes mellitus. Ear and Hearing, 38(6), 724-735.
Stamper, G. C. & Johnson, T. A. (2015). Auditory function in normal-hearing, noise-exposed human ears. Ear and Hearing, 36(2), 172-184.
Stephens, D., Zhao, F., & Kennedy, V. (2003). Is there an association between noise exposure and King Kopetzky syndrome? Noise and Health, 5(20), 55-62.
Valderrama, J. T., Beach, E. F., Yeend, I., Sharma, M., Van Dun, B., & Dillon, H. (2018). Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hearing Research, 365, 36-48.
World Health Organization. (2015, March 10). 1.1 Billion People at Risk of Hearing Loss. World Health Organization. Retrieved from https://www.who.int/vietnam/news/detail/10-03-2015-1.1-billion-people-at-risk-of-hearing-loss.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||