AbstractPurpose The study aims to address the gap in understanding the relationship between type 2 diabetes mellitus (T2DM) and vestibular dysfunction, particularly focusing on how T2DM may affect the tuning of the vestibular system. Specifically, the investigation aims to explore the impact of stimulus parameters, particularly stimulus frequency, on ocular vestibular evoked myogenic potentials (oVEMP) in individuals with T2DM.
Methods A case-control study design was used, utilizing non-probability sampling. All participants underwent routine audiological and vestibular evaluations before inclusion in the study. oVEMP’s were compared across 40 healthy individuals (group A) and 40 individuals with T2DM (group B) at 500 Hz, 1 KHz, and 2 KHz.
Results In group A, all 80 participants exhibited oVEMP responses across all frequencies in both ears, while in group B, consisting of individuals with T2DM, response rates were slightly lower, ranging from 70% to 77.5% across frequencies. Statistical analysis revealed significant amplitude differences at all frequencies tested in type 2 diabetic ears compared to healthy ears, although amplitude remained consistent within type 2 diabetic ears. Latencies (first negative [N1] and first positive [P1]) were consistent across groups, with delays observed in P1 latency, particularly noticeable at higher frequencies, both in healthy and type 2 diabetic ears. Gender didn't affect oVEMP frequency dynamics in any of the groups.
Conclusion Diabetes may create an impact in the functioning of otolith organs, the vestibular branch of eighth cranial nerve, and their innervations. VEMP evaluates both the sacculo-collic and utriculo-ocular pathways to detect changes in the functions of vestibular organs. Therefore, it's crucial to consider diabetes when conducting vestibular diagnosis or treatment.
INTRODUCTIONDiabetes is a chronic metabolic disease with elevated blood glucose levels, which over time harms the heart, blood vessels, eyes, kidneys, and nerves (World Health Organization, 2016). Type 1 diabetes mellitus and type 2 diabetes mellitus (T2DM) are the two subtypes. According to the International Classification of Diseases 11th Revision, T2DM is a disorder in which the kidneys produce a lot of urine and the body’s glucose levels are out of control which arises when the body doesn’t create enough insulin or use it appropriately.
The adverse effects of hyperglycemia are categorized as macrovascular (stroke, peripheral artery disease, and heart disease) and microvascular (aneurysms & thrombosis, diabetic nephropathy, neuropathy, and retinopathy). According to Ward et al.(2015), people with type 2 diabetes are 70% more likely to have inner ear problems than people without the disease. Additionally, compared to individuals with a medical history of less than 5 years, the frequency significantly increases among those with an illness duration of more than 6 years (Ward et al., 2015). Diabetes can trigger difficulties in hearing and vestibular function (Chiles et al., 2014) because the cochlea and the vestibular apparatus share a nerve and blood supply. Patients with type 2 diabetes who have the disease for a longer period of time have a higher incidence of vestibular dysfunction (Agrawal et al., 2009; Ward et al., 2015). In animal models of diabetes, both morphological and physiological alterations have been observed in the peripheral vestibular apparatus. Myers and colleagues discovered structural changes, including an excess of extracellular matrix and increased lysosomes and lipid droplets, primarily in the utricle and saccule, attributed to metabolic stress (Myers & Ross, 1987; Myers et al., 1985). These changes hindered the diffusion of essential substances, potentially leading to hair cell degeneration, particularly notable in the saccule, suggesting its heightened vulnerability to diabetic pathology (Myers & Ross, 1987). Additionally, diabetic rats exhibited disruptions in the myelin sheath of the vestibulocochlear nerve, while mice with type 2 diabetes showed delayed evoked potential responses and reduced amplitudes, indicating impaired vestibular function (Myers, 1998; Myers et al., 1999). Perez and colleagues investigated the physiological shifts in the vestibular organ caused by diabetes in mice with diet-induced type 2 diabetes. They observed delays in the latency of evoked potential responses, decreased amplitudes, and elevated thresholds compared to control mice. The presence of abbreviated vestibular evoked potentials in response to linear and angular accelerations demonstrated the impact of diabetes on vestibular function (Perez et al., 2001).
An electromyographic response to sound stimuli is generated from the utricular region of the opposite side and the superior vestibular nerve functions, known as ocular vestibular-evoked myogenic potential (VEMP) (Colebatch et al., 2016; Rosengren & Kingma, 2013). VEMP has been an integral part of the vestibular test battery (Colebatch & Halmagyi, 1992). Extraocular muscles get the vestibuloocular reflex (VOR) from the otoliths. Eye movements and peri-ocular short-latency potentials were recorded simultaneously and showed no anatomical association with the origin of surface potentials, suggesting that the observed potentials could not be caused only by eye movements. El-seady et al.(2022) investigated the potential use of ocular VEMP in the analysis of vestibular problems in individuals with T2DM. The authors found that there were significant discrepancies between the first negative (N1) and first positive (P1) latencies of individuals who suffered from diabetic peripheral neuropathy (DPN) and those who did not suffer from this condition.
Anatomical and structural analyses have demonstrated that the otolith organs have a lower mechanical resonance (Uzun-Coruhlu et al., 2007), which could also explain the frequency tuning at 500 Hz. According to Todd et al.(2000), the frequency tuning features of VEMP is potentially modelled by a 2nd order mechanical system that is comprised of mass and high stiffness. According to Park et al.(2010), there is no substantial difference between frequencies of ocular VEMP (oVEMP) tuning in normal individuals. Rauch et al.(2004) were the first research studies to establish that VEMP tuning may be affected by disorders that have an effect on the mechanics of the inner ear. Patients diagnosed with Meniere’s disease had higher amplitudes and lower thresholds at a frequency of 1,000 Hz, as opposed to the frequency of 500 Hz, which is typical for the majority of participants in the control group (Winters et al., 2011). Watson et al.(2000) showed that patients with semicircular canal dehiscence (SCD) had unusually elevated peak amplitudes of the VEMP, as well as abnormally low thresholds. This suggests that the vestibular system demonstrates a phenomenon known as "frequency tuning", which is influenced by age. In young, healthy adults, the most significant VEMP responses typically occur at approximately 500 Hz. However, in older adults aged 60 and above, the predominant VEMP responses are more commonly observed at frequencies of 750 Hz and 1,000 Hz. Consequently, it is advisable to test tone burst frequencies of 750 Hz and 1,000 Hz in addition to 500 Hz if responses are absent at the latter frequency (Piker et al., 2013). Similar to vestibular disorders and the influence of age, conditions such as T2DM can also impact the responses of oVEMP. Additionally, there is insufficient data regarding how oVEMP responses vary across frequencies in individuals with T2DM.
Previous studies have primarily focused on assessing otolith dysfunction and conducting audio-vestibular profiling in individuals with T2DM, yet there remains a notable gap in understanding the specific mechanisms underlying vestibular dysfunction in this population (DiLiberto et al., 2024; Kumar et al., 2021). Consequently, it remains unclear whether T2DM affects the tuning of the vestibular system. Hence, there is a critical need to investigate the impact of oVEMP in individuals with T2DM. To address these knowledge gaps, the current study was conducted to explore the effects of various stimulus parameters, particularly frequency, on air-conduction induced oVEMP in individuals diagnosed with T2DM. Our approach involved identifying the VEMP tuning profile by simultaneously recording oVEMP in healthy participants and comparing the results with those from individuals with T2DM.
The null hypothesis states that there will be no significant statistical distinction in altered frequency dynamics using air conduction-induced oVEMPs between individuals with T2DM and those without. It also suggests that there will be no significant statistical difference across gender or in relation to the duration of T2DM. The alternative hypothesis proposes that there will be significant statistical differences in altered frequency dynamics using oVEMPs among individuals with T2DM compared to those without, across gender, and concerning the duration of T2DM.
MATERIALS AND METHODSSubjectsA non-probability sampling technique was used for the selection of the participants and all were subjected to routine audiological and vestibular evaluation before inclusion. Non-probability sampling involves selecting individuals or items for a sample without relying on random selection. Instead, researchers use their judgment, convenience, or specific criteria to choose participants or items (Kumar, 2018). Altered frequency dynamics (500 Hz, 1,000 Hz, and 2,000 Hz) of oVEMP were assessed. Group A consisted of 40 healthy individuals (n = 80). Group B consisted of 40 subjects with T2DM (n = 80). Group A encompasses individuals aged 30 to 50 without diabetes, verified to have no history of trauma, stroke, middle ear issues, or surgeries, and possessing normal hearing thresholds in the octave frequency range of 250 Hz to 8,000 Hz within 25 dB hearing level (HL). This cohort acts as the control for the study. Group B includes individuals aged 30 to 50 diagnosed with T2DM for at least 1 year, devoid of accompanying neurological conditions mentioned earlier. Like group A, participants in group B have normal hearing thresholds in the octave frequency range of 250 Hz to 8,000 Hz within 25 dB HL. Exclusion criteria were applied uniformly to maintain study integrity, excluding those with type 1 diabetes mellitus, participants taking non-diabetes or hypertension-related ototoxic medications, and individuals with specific vestibular disorders.
EquipmentThe following equipment was used in the study: the pure-tone thresholds, and speech recognition thresholds were all determined using an Otometrics Madsen Astera2 a two-channel diagnostic audiometer (Natus Medical Incorporated, Pleasanton, CA, USA). Tympanometry and reflexometry were performed using the GSI Tymp Star Pro (Grason-Stadler, Colorado Springs, CO, USA). oVEMP was performed using (Neuro Audio) AEP, complete, DI 200300 (Neurosoft Ltd., Minsk, Belarus).
oVEMP recording500 Hz, 1,000 Hz, and 2,000 Hz air-conducted tone bursts were presented using calibrated ER3A insert earphones at 127 dB sound pressure level in alternating polarity with the following stimulus profile: 2 ms peak time, 0 ms plateau duration, and 2 ms fall time. Averaging at 5.1/s provided consistency.
The subjects were seated in a sitting upright position and were instructed to maintain an upward gaze of approximately 30 to 35 degrees, on a pre-marked visual target. Surface electromyography electrodes were used to record responses from behind both eyes after thorough cleansing of the skin beneath the eyes with rubbing alcohol/NUPREP skin gel. The active (+ve) electrode was positioned about 1 cm below the lower eyelid on the infra-orbital ridge, and the reference (-ve) electrode was placed around 3 cm below the lower eyelid in each eye. The common electrode was placed on the forehead. Because the recorded potentials are minimal, the electrode leads may need to be shielded, with the shields connected to the subject’s ground electrode. In all trials, the absolute and inter-electrode impedance was maintained below 5 KΩ and 2 KΩ respectively. The subjects were instructed to look up at a distant target in the midline, the electrodes were aligned with the center of the pupil. To allow for this close electrode insertion, the electrode is then stuck with micropore tape over the electrode, making sure that no electrical bridge forms between the two electrodes (Kumar et al., 2021).
MeasuresOn oVEMP, the N1 and P1 peaks were marked on the waveform of three frequencies (500 Hz, 1,000 Hz, and 2,000 Hz). The waveform latency, peak-to-peak amplitude, and amplitude ratio were analyzed. The response rates were calculated as the percentage of participants exhibiting present oVEMP responses out of the total number of participants tested. This calculation involves dividing the number of participants with present oVEMP responses by the total number of participants tested and multiplying by 100 to obtain the percentage.
Data analysisStatistical analysis including descriptive and inferential statistics was performed for data analysis. The analyzed data was interpreted accordingly. The obtained data was tabulated using Statistical Package for the Social Sciences (SPSS) software version 26 from IBM Corp. (Armonk, NY, USA) in 2019, and subjected to analysis. Descriptive statistics was done to estimate the mean and standard deviation of age along with the number of participants based on gender. Inferential statistics was carried out to measure the variables appropriately. p-value < 0.05 was considered a statistically significant difference.
Independent’ t-test was done to compare the results of altered frequency dynamics of oVEMP in individuals with T2DM. Paired sample ‘t’ test was administered to compare the altered frequency dynamics of oVEMP among individuals with T2DM and without DM. One-way repeated measures analysis of variance was used to check the effect of gender on altered frequency dynamics of oVEMP in individuals with T2DM.
RESULTSWaveform of 500 Hz, 1,000 Hz, and 2,000 Hz tone burst oVEMP obtained from healthy ears and ears with T2DM is shown in Figure 1. Overall mean and standard deviation for the peak amplitude and latencies of three frequency tone burst oVEMP obtained from healthy ears and ears with T2DM are summarized in Table 1.
oVEMP response prevalenceIn group A, all the participants (n = 80) had oVEMP responses and the response rate was 100% for three frequencies in both ears. In group B, oVEMP response rates of 77.5% (n = 62), 77.5% (n = 62), and 72.5% (n = 58) were obtained for 500 Hz, 1,000 Hz, and 2,000 Hz, respectively. Consequently, oVEMP responses were absent in 22.5% (n = 18), 22.5% (n = 18), and 27.5% (n = 22) at 500 Hz, 1,000 Hz, and 2,000 Hz, respectively (Table 1).
oVEMP amplitudesIn individuals with T2DM, there were statistically significant differences in amplitude among frequencies at 500 Hz (p = 0.427), 1,000 Hz (p = 0.038), and 2,000 Hz (p = 0.024). However, when comparing amplitude between healthy ears and ears affected by T2DM across frequencies, there was no significant change at 500 Hz (p = 0.514) and 1,000 Hz (p = 0.931), but there was a significant difference at 2,000 Hz (p = 0.0456) (Figure 2).
oVEMP amplitude ratioThe amplitude ratio was determined by dividing one frequency’s mean amplitude by another. It was tested in both healthy individuals and T2DM individuals across three frequencies. In both groups, 500/1,000 Hz did not show any difference the mean 1,000 Hz/2,000 Hz amplitude ratio was raised whereas 2,000 Hz/500 Hz was reduced respectively.
oVEMP latenciesBoth N1 and P1 latencies in response to the frequencies were matched among both the groups, revealing there was a statistically significant effect on both N1 and P1 latency at 500 Hz (p = 0.000), 1,000 Hz (p = 0.000), 2,000 Hz (p = 0.000) and 500 Hz (p = 0.000), 1,000 Hz (p = 0.001), 2,000 Hz (p = 0.000) for healthy ears and ears with T2DM respectively. As the frequency rises in individuals with T2DM, the latencies of N1 and P1 also increase across most frequencies except at 2,000 Hz. Conversely, in healthy individuals, as the frequency increases, N1 and P1 latencies occur earlier. The mean and standard deviation for latencies has been listed above in Table 1, Figure 3.
Gender effect on oVEMP responsesGender effect was studied on both groups across three different frequencies. Group A had 23 men and 17 women, while group B had 42 men and 18 women. oVEMP did not vary for any of the three frequencies (500 Hz, 1,000 Hz, and 2,000 Hz) between genders (p > 0.05). According to the null hypotheses, the oVEMP responses had no statistically significant impact on gender (Table 2).
DISCUSSIONSThe literature regarding Altered frequency dynamics of oVEMP was carried out in normal individuals (Janky & Shepard, 2009), Meniere’s disease (Singh & Barman, 2016), Semi-circular canal dehiscence (Zhang et al., 2012) and elevated intracranial pressure individuals (Gürkov et al., 2016). Several researches have revealed an association between diabetes mellitus and vestibular dysfunction; however, the most compromised part is yet unclear. Recent advancements in the vestibular test battery now enable the individual evaluation of each vestibular end-organ, all of which are believed to be impaired in T2DM.
oVEMP response prevalenceIn the current investigation, in group A, oVEMP responses were found in all ears (n = 80) with 100% response rate across all three frequencies indicating normal vestibular system functioning. In group B, 77.5% (n = 62), 77.5% (n = 62), and 70% (n = 58) of ears responded to 500 Hz, 1,000 Hz, and 2,000 Hz, respectively. Likewise, 22.5% (n = 9), 22.5% (n = 9), and 30% (n = 11) of persons exhibited absent oVEMP responses at 500 Hz, 1,000 Hz, and 2,000 Hz respectively. Response predominance was dramatically condensed in patients with T2DM, indicating impaired vestibular system function.
Agrawal et al.(2010) investigated the influence of vestibular dysfunction on the risk of falling for diabetic individuals. It was found that there was a high incidence of absent oVEMP as a result of neurovascular disruption as seen in nerve conduction studies. In addition, he concluded that individuals with severe diabetic neuropathy had a 76% likelihood of having absent oVEMP. Kanumuri et al.(2018) found that DM condition resulted in a bilateral lack of oVEMP responses in 10% of those with T2DM that was present for more than 5 years.
oVEMP amplitude – N1-P1In the present study, the mean of the N1-P1 amplitude (µv) was found to be reduced in both group A (n = 80) and group B (n = 80). Statistically significant difference was noted in N1-P1 (µv) amplitude across frequencies (500 Hz, 1,000 Hz, and 2,000 Hz) in ears with T2DM. Also, there was no significant difference noted among healthy ears and T2DM ears.
Studies by Ward et al.(2015) also showed very similar results. He tried to functionally localize vestibular impairments in T2DM and demonstrated that oVEMP peak-to-peak amplitude and n1 amplitude were not reduced in the group who had T2DM. Another supported evidence by Kalkan et al.(2018) did not show any changes in their oVEMP of T2DM with respect to amplitude values when compared to healthy controls. In contrast, Kamali et al.(2013) found reduced oVEMP inter-peak amplitudes in T2DM patients varied across the control group and observed that in T2DM, vestibular deficits were more significant than in healthy people of the same age.
oVEMP latencies – N1 and P1With respect to latency parameters, in the current study N1 and P1 latencies (ms) are found to be prolonged as the frequency increased in both the groups. In group B, N1 and P1 latencies across frequencies (500 Hz, 1,000 Hz, and 2,000 Hz) are observed to be earlier when compared to healthy ears. A significant difference is noted in N1 and P1 latency across frequencies in ears with T2DM. Similarly, between healthy ears and ears with T2DM a substantial difference was observed in P1 and N1 latency across frequencies.
Perez et al.(2001) with respect to latencies stated that oVEMP in patients with T2DM had delayed latency responses when compared to healthy controls. Another evidence by Rigon et al.(2007) stated that T2DM patients had delayed P1 and N1 latency responses compared to healthy individuals. Two uncertainties why oVEMP is impacted in T2DM patients with delayed P1 and N1 latencies are nerve bundles with thick or thin myelinated sheath are degraded and diabetic neuropathy causes Wallerian degeneration, segmental or paranodal demyelination, and endoneural connective tissue proliferation (Malik et al., 1993). Similar to type 1, T2DM is characterized by main Schwann cell abnormalities. Cervical VEMP compensation or recruiting may be quicker than oVEMP.
oVEMP morphology and replicabilityMorphology and the ability to replicate are the defining characteristics of the waveforms. The morphology is determined by the structure, amplitude, or phase orientations, as well as the duration, of the motor unit potentials, which are regarded to be responses. The replicability involves having precise, real-time, and multi-channel control over the duplicate of a waveform response.
In the present study, both the waveform morphology and the replicability of T2DM ears were shown poorer than those of healthy ears, particularly at higher frequencies. This was evidently true for T2DM ears. In support of the result, Kanumuri et al.(2018) reported that persons with T2DM were seen to have weak waveform morphology.
oVEMP frequency tuning sensitivityThe depiction of the elastic and inertial components, the mechanical resonance of each stereocilia, and the electrical tuning of the hair cells in the vestibular end organs are what make up the frequency tuning of the two otolith organs (Welgampola & Colebatch, 2001).
According to the outcomes of the present study, 500 Hz is the optimal frequency for capturing the maximal oVEMP amplitudes across frequencies in both healthy ears and T2DM. According to Piker et al.(2013), the best frequency for eliciting cervical VEMP responses is between 500 and 1,000 Hz. This statement was replicated by their analyses.
Gender effect on oVEMPThe third objective was to estimate the effect of gender on individuals with T2DM. There was no noteworthy difference in oVEMP responses across male and female groups. The mean N1 latency, P1 latency, and N1-P1 interval did not vary significantly between male and female genders. In this study, altered frequency dynamics of oVEMP did not produce a gender-based difference.
Similar results were reported by Sung et al.(2011). He stated that the N1 latency, P1 latency, and N1–P1 interval of oVEMP did not change substantially between male and female adults, demonstrating that the path length of the VOR is not affected by gender. In contrast, Carnaúba et al.(2011) stated that the oVEMP amplitude of men was substantially greater than that of females, most likely owing to a structural variation. oVEMP recorded from behind the eyes when individuals gaze upward; the inferior oblique muscle seems to dominate. Male’s extraocular muscles had considerably greater mean diameters morphologically than females (Özgen & Aydingöz, 2000). As a result, men’s thicker inferior oblique muscles are thicker and contract more synchronous motor units when they look upward. Hence compound action potentials have a greater amplitude in males. Since males have more muscle mass than females, this implies that differences in oVEMP amplitude may be attributed to anatomical differences between genders. Many individuals believe that diabetes-induced inner ear dysfunction can lead to damage in different parts of the vestibular system, including nerves and ganglia, as a secondary effect. Evaluating VEMPs helps gauge the function of critical pathways and aids in identifying dysfunction, thereby preventing potential deterioration of vestibular organ health. VEMPs are a valuable diagnostic tool for various vestibular disorders and should account for diabetes as a significant factor in assessment and treatment plans. It is essential to analyze duration effects across different frequencies, particularly focusing on mid-octave frequencies like 750 Hz and 1,500 Hz. Additional vestibular assessment methods such as video-nystagmography and video-head impulse tests provide further understanding of the level of damage associated with T2DM.
NotesEthical Statement Written informed consent was obtained from the study participants, and Ethics Committee of SRM Medical College Hospital and research centre approved the study proposal (approval number: 3182/IEC/2021). Declaration of Conflicting Interests There is no potential conflict of interest disclosed by the authors. Funding This study received no funding from government bodies, the public, or any other organizations. Author Contributions Conceptualization: Maanasa Pallikonda Seshagiri, Akshya Purushothaman, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Data collection: Maanasa Pallikonda Seshagiri, Akshya Purushothaman, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Formal analysis: Maanasa Pallikonda Seshagiri, Akshya Purushothaman, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Writing—original draft: Maanasa Pallikonda Seshagiri, Akshya Purushothaman, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Writing—review & editing: Maanasa Pallikonda Seshagiri, Akshya Purushothaman, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Approval of final manuscript: all authors. AcknowledgmentsThe authors are grateful to the Department of Audiology and Speech Language Pathology at the SRM Institute of Science and Technology for providing the tools needed to carry out the study. Additionally, the study’s participants at large are acknowledged.
Figure 1.Waveform of 500 HZ, 1,000 HZ, and 2,000 HZ tone burst ocular vestibular evoked myogenic potentials from healthy ears (A) and ears with type 2 diabetes mellitus (B). 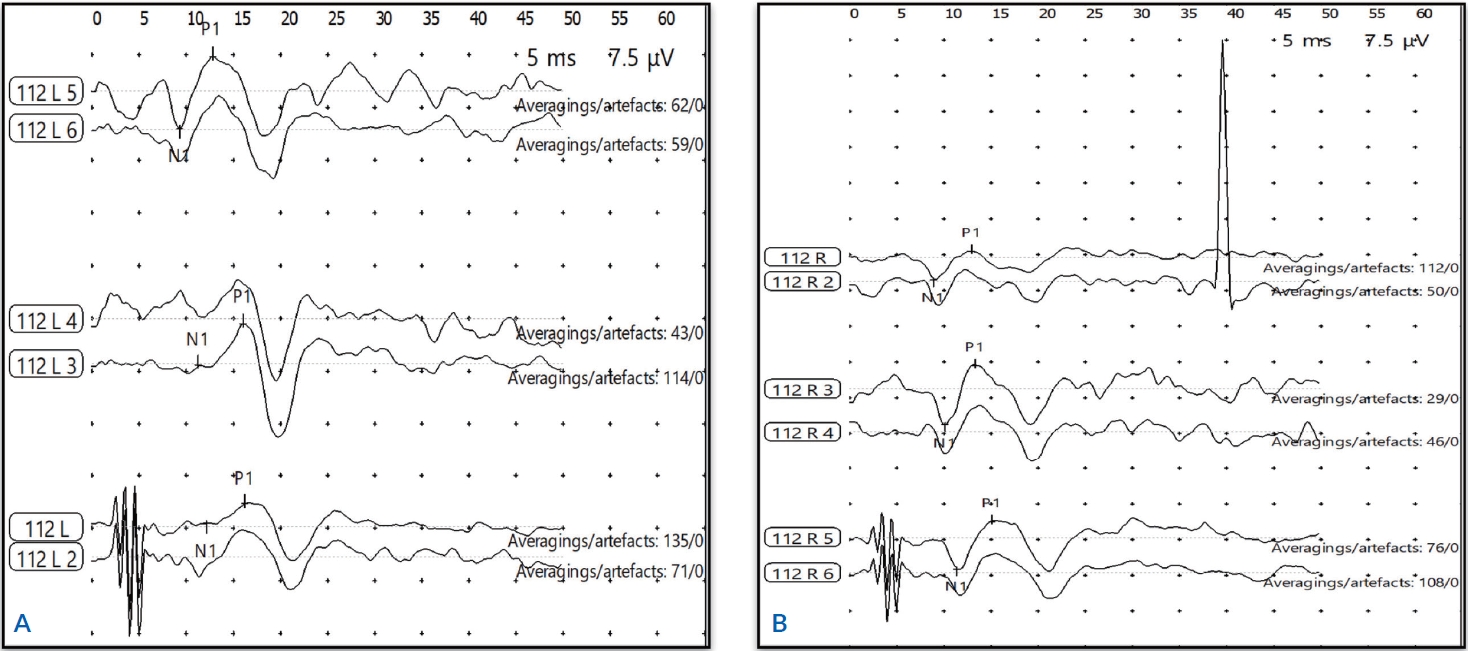
Figure 2.Overall mean amplitudes of 500 Hz, 1,000 Hz, and 2,000 Hz tone burst ocular vestibular evoked myogenic potential obtained from healthy ears and ears with type 2 diabetes mellitus. N1: first negative, P1: first positive. 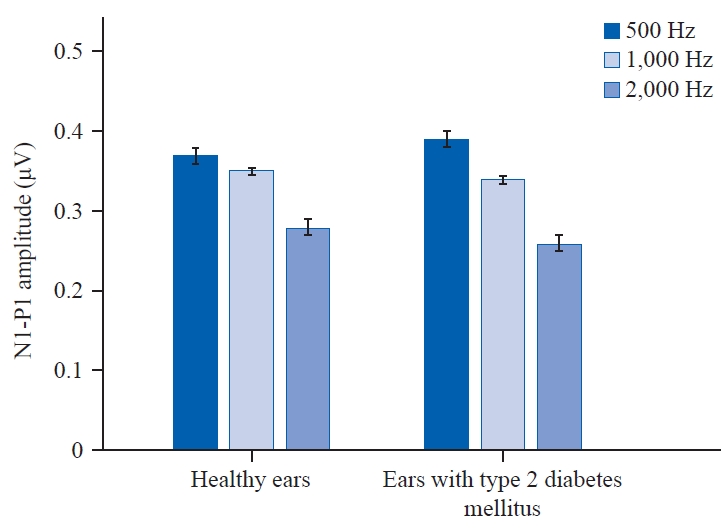
Figure 3.Peak latency of 500 Hz, 1,000 Hz, and 2,000 Hz tone burst ocular vestibular evoked myogenic potential obtained from healthy ears and ears with type 2 diabetes mellitus. P1: first positive, N1: first negative. 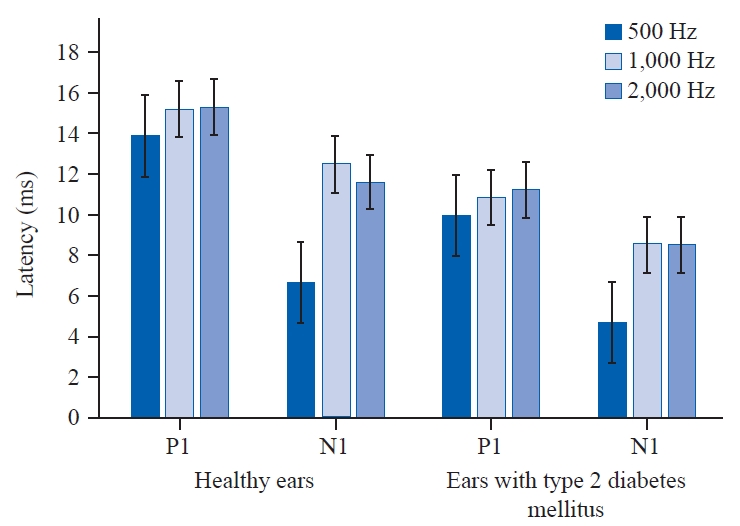
Table 1.Overall mean and standard deviation for the peak amplitude and latencies of 500 Hz, 1,000 Hz, and 2,000 Hz tone burst oVEMP obtained from healthy ears and ears with type 2 diabetes mellitus Table 2.Comparison of gender effect on peak latency and amplitudes of 500 Hz, 1,000 Hz, and 2,000 Hz tone burst ocular VEMP obtained from type 2 diabetes mellitus ears
REFERENCESAgrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C., & Minor, L. B. (2009). Disorders of balance and vestibular function in US adults: Data from the National Health and Nutrition Examination Survey, 2001-2004. Archives of Internal Medicine, 169(10), 938-944.
Agrawal, Y., Carey, J. P., Della Santina, C. C., Schubert, M. C., & Minor, L. B. (2010). Diabetes, vestibular dysfunction, and falls: Analyses from the National Health and Nutrition Examination Survey. Otology and Neurotology, 31(9), 1445-1450.
Carnaúba, A. T., Farias, V. V., Santos, N., Oliveira, A. C., Rodrigues, R. G., & Menezes, P. de. L. (2011). Influence of gender on the vestibular evoked myogenic potential. Brazilian Journal of Otorhinolaryngology, 77(2), 245-248.
Chiles, N. S., Phillips, C. L., Volpato, S., Bandinelli, S., Ferrucci, L., Guralnik, J. M., et al. (2014). Diabetes, peripheral neuropathy, and lower-extremity function. Journal of Diabetes and its Complications, 28(1), 91-95.
Colebatch, J. G. & Halmagyi, G. M. (1992). Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology, 42(8), 1635-1636.
Colebatch, J. G., Rosengren, S. M., & Welgampola, M. S. (2016). Vestibular-evoked myogenic potentials. Handbook of Clinical Neurology, 137, 133-155.
DiLiberto, F. E., Kamath, H. E. R., Olson, M. L., Cherchi, M., Helminski, J. O., & Schubert, M. C. (2024). When, where, and why should we look for vestibular dysfunction in people with diabetes mellitus? Frontiers in Rehabilitation Sciences, 4, 1306010.
El-seady, I. Y., Abd El-Tawab, M. M., El-Shawaf, W. I., & El-Sehrawy, A. A. (2022). Role of vestibular evoked myogenic potential (VEMP) in diagnosis of vestibular abnormalities in patients with type 2 diabetes mellitus. The Egyptian Journal of Internal Medicine, 34(1), 36.
Gürkov, R., Speierer, G., Wittwer, L., & Kalla, R. (2016). Effect of elevated intracranial pressure on amplitudes and frequency tuning of ocular vestibular evoked myogenic potentials elicited by bone-conducted vibration. Ear and Hearing, 37(6), e409-e413.
Janky, K. L. & Shepard, N. (2009). Vestibular evoked myogenic potential (VEMP) testing: Normative threshold response curves and effects of age. Journal of the American Academy of Audiology, 20(8), 514-522.
Kalkan, M., Bayram, A., Gökay, F., Cura, H. S., & Mutlu, C. (2018). Assessment of vestibular-evoked myogenic potentials and video head impulse test in type 2 diabetes mellitus patients with or without polyneuropathy. European Archives of Oto-Rhino-Laryngology, 275(3), 719-724.
Kamali, B., Hajiabolhassan, F., Fatahi, J., Nasli Esfahani, E., Sarrafzadeh, J., & Faghihzadeh, S. (2013). Effects of diabetes mellitus type Ι with or without neuropathy on vestibular evoked myogenic potentials. Acta Medica Iranica, 51(2), 107-112.
Kanumuri, S., Chaitanya, K. V., Nara, J., & Reddy, K. V. K. (2018). Role of cervical vestibular-evoked myogenic potentials in evaluating vestibular dysfunction in patients with type II diabetes mellitus: A prospective institutional study. Indian Journal of Otology, 24(2), 105-108.
Kumar, P., Singh, N. K., Apeksha, K., Ghosh, V., Kumar, R. R., & Kumar Muthaiah, B. (2021). Auditory and vestibular functioning in individuals with type-2 diabetes mellitus: A systematic review. International Archives of Otorhinolaryngology, 26, e281-e288.
Kumar, R. (2018). Research Methodology: A Step-by-Step Guide for Beginners. (5th ed.), (pp.1-528). Melbourne: SAGE Publications Ltd.
Malik, R. A., Tesfaye, S., Thompson, S. D., Veves, A., Sharma, A. K., Boulton, A. J., et al. (1993). Endoneurial localisation of microvascular damage in human diabetic neuropathy. Diabetologia, 36(5), 454-459.
Myers, S. F. (1998). Myelin-sheath abnormalities in the vestibular nerves of chronically diabetic rats. Otolaryngology-Head and Neck Surgery, 119(5), 432-438.
Myers, S. F. & Ross, M. D. (1987). Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus. II. Connective tissue and neuroepithelial pathology. Acta Oto-Laryngologica, 104(1-2), 40-49.
Myers, S. F., Ross, M. D., Jokelainen, P., Graham, M. D., & McClatchey, K. D. (1985). Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus. I. Microvascular changes. Acta Oto-Laryngologica, 100(5-6), 351-364.
Myers, S. F., Tormey, M. C., & Akl, S. (1999). Morphometric analysis of horizontal canal nerves of chronically diabetic rats. Otolaryngology-Head and Neck Surgery, 120(2), 174-179.
Özgen, A. & Aydingöz, Ü. (2000). Normative measurements of orbital structures using MRI. Journal of Computer Assisted Tomography, 24(3), 493-496.
Park, H. J., Lee, I. S., Shin, J. E., Lee, Y. J., & Park, M. S. (2010). Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clinical Neurophysiology, 121(1), 85-89.
Perez, R., Ziv, E., Freeman, S., Sichel, J. Y., & Sohmer, H. (2001). Vestibular end-organ impairment in an animal model of type 2 diabetes mellitus. The Laryngoscope, 111(1), 110-113.
Piker, E. G., Jacobson, G. P., Burkard, R. F., McCaslin, D. L., & Hood, L. J. (2013). Effects of age on the tuning of the cVEMP and oVEMP. Ear and Hearing, 34(6), e65-e73.
Rauch, S. D., Zhou, G., Kujawa, S. G., Guinan, J. J., & Herrmann, B. S. (2004). Vestibular evoked myogenic potentials show altered tuning in patients with Meniere’s disease. Otology and Neurotology, 25(3), 333-338.
Rigon, R., Rossi, A. G., & Cóser, P. L. (2007). Otoneurologic findings in type 1 diabetes mellitus patients. Brazilian Journal of Otorhinolaryngology, 73(1), 100-105.
Rosengren, S. M. & Kingma, H. (2013). New perspectives on vestibular evoked myogenic potentials. Current Opinion in Neurology, 26(1), 74-80.
Singh, N. K. & Barman, A. (2016). Utility of the frequency tuning measure of oVEMP in differentiating Meniere’s disease from BPPV. Journal of the American Academy of Audiology, 27(9), 764-777.
Sung, P. H., Cheng, P. W., & Young, Y. H. (2011). Effect of gender on ocular vestibular-evoked myogenic potentials via various stimulation modes. Clinical Neurophysiology, 122(1), 183-187.
Todd, N. P., Cody, F. W., & Banks, J. R. (2000). A saccular origin of frequency tuning in myogenic vestibular evoked potentials?: Implications for human responses to loud sounds. Hearing Research, 141(1-2), 180-188.
Uzun-Coruhlu, H., Curthoys, I. S., & Jones, A. S. (2007). Attachment of the utricular and saccular maculae to the temporal bone. Hearing Research, 233(1-2), 77-85.
Ward, B. K., Wenzel, A., Kalyani, R. R., Agrawal, Y., Feng, A. L., Polydefkis, M., et al. (2015). Characterization of vestibulopathy in individuals with type 2 diabetes mellitus. Otolaryngology-Head and Neck Surgery, 153(1), 112-118.
Watson, S. R., Halmagyi, G. M., & Colebatch, J. G. (2000). Vestibular hypersensitivity to sound (Tullio phenomenon): Structural and functional assessment. Neurology, 54(3), 722-728.
Welgampola, M. S. & Colebatch, J. G. (2001). Vestibulocollic reflexes: Normal values and the effect of age. Clinical Neurophysiology, 112(11), 1971-1979.
Winters, S. M., Berg, I. T., Grolman, W., & Klis, S. F. (2011). Ocular vestibular evoked myogenic potentials: Frequency tuning to air-conducted acoustic stimuli in healthy subjects and Ménière's disease. Audiology and Neurotology, 17(1), 12-19.
World Health Organization. (2016, April 21). Global Report on Diabetes. World Health Organization. Retrieved from https://www.who.int/publications/i/item/9789241565257.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||